



Table of Contents: 2024 JULY–AUGUST No. 459
The ClinicalTrials.gov PRS Beta Will Soon Become the Primary Website for Protocol Registration. NLM Tech Bull. 2024 Jul-Aug;(459):e2.
On August 28, 2024, the Protocol Registration and Results System (PRS) Beta will become the primary website for protocol registration and management of your list of study records. New features of the Modernized PRS website include:
In addition, data submitters can use the modernized Record Summary page to view a summary of the quality-assurance/quality-control review comments as well as access the comments directly in the relevant protocol registration modules.
These changes are currently available in the modernized PRS Test. To learn more about these and other new features of the Modernized PRS prior to the transition, log into PRS Test.
After the Modernized PRS becomes the primary website, users can seamlessly transition to the Classic PRS to manage some of their records and to enter study results. By default, records containing only the Protocol Section will open in the Modernized PRS. Some functions of the Classic PRS are still being refined within the Modernized PRS as development continues. However, you can easily transition between the classic and modernized websites. For example, to correct certain validation errors, you may have to move from the Modernized PRS to the Classic PRS website. Save your changes in one website before moving to the other. Once saved, data entered in one website will be available in the other.
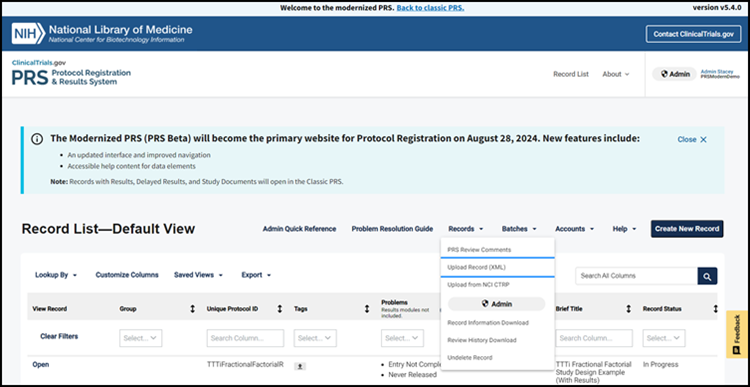
Functions not yet available in the Modernized PRS such as uploading a record and records with Results, Delayed Results, and Study Documents will open in the Classic PRS (Figure 1) directly from the modernized website. These sections are still under development and will be available in the modernized website in the future. Similarly, certain links—such as those used to manage accounts and access certain help materials—will take users back to the Classic PRS until these items are available on the modernized website.
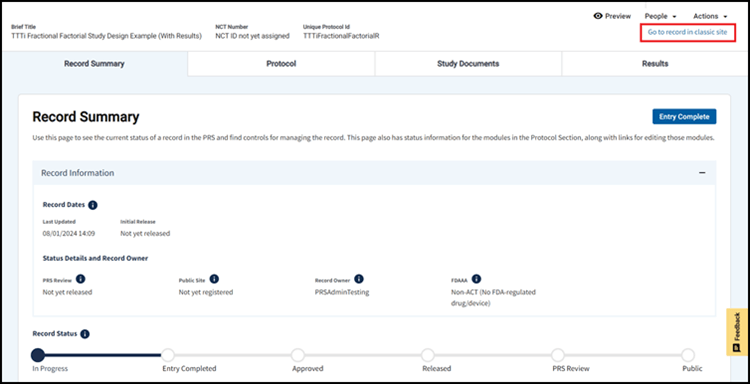
The Modernized PRS website will continue to be expanded and improved, and to ensure an optimal user experience, your feedback is essential. Please click the yellow Feedback button in the lower right corner of the screen (Figure 2) to let us know what you think of the Modernized PRS. More information about the transition to the Modernized PRS can be found in the Modernization Transition Top Questions. For registration and results submission questions, please email us at register@clinicaltrials.gov.